About the project
Ascilion's sampling system enables new biomedical research and future diagnostic opportunities by collecting sufficiently large
volumes of dermal interstitial fluid for subsequent analysis in an operator and patient friendly way. The PELSA system samples and collects
dermal interstitial fluid using hollow microneedles and a mild vacuum. The needle chip has a built-in capillary system and a fluidic storage
reservoir for collection of the fluid
Read more here
Fun fact!
Sampling of dermal Interstitial Fluid (dISF)
Project description
Ascilion was founded in 2012 with a mission to solve the problem of sampling dermal interstitial
fluid in a timely and pain-free way. A team of engineers with a thorough understanding of MEMS technology and microfluidics took on the challenge of solving
what turned out to be a very complex problem. Founded on the premise of radically improving healthcare, the team set out to develop hollow
microneedles that can sample dermal interstitial fluid in a pain-free and easy to use way. After almost 10 years of development, the company is entering
into numerous clinical trials with the aim of characterizing both the system and dISF itself.
Dermal interstitial fluid, as opposed to the bloodstream, is not under positive pressure. The fluid isn't easy to sample. All parts of a system
intended for sampling of dermal interstitial fluid need to be highly optimized and work in conjunction for an efficient sampling process.
Ascilion has spent over a decade refining every part of the system from the needle chip to the instrument controlling the process via the method
for how the parts fit together with maintained sterility.
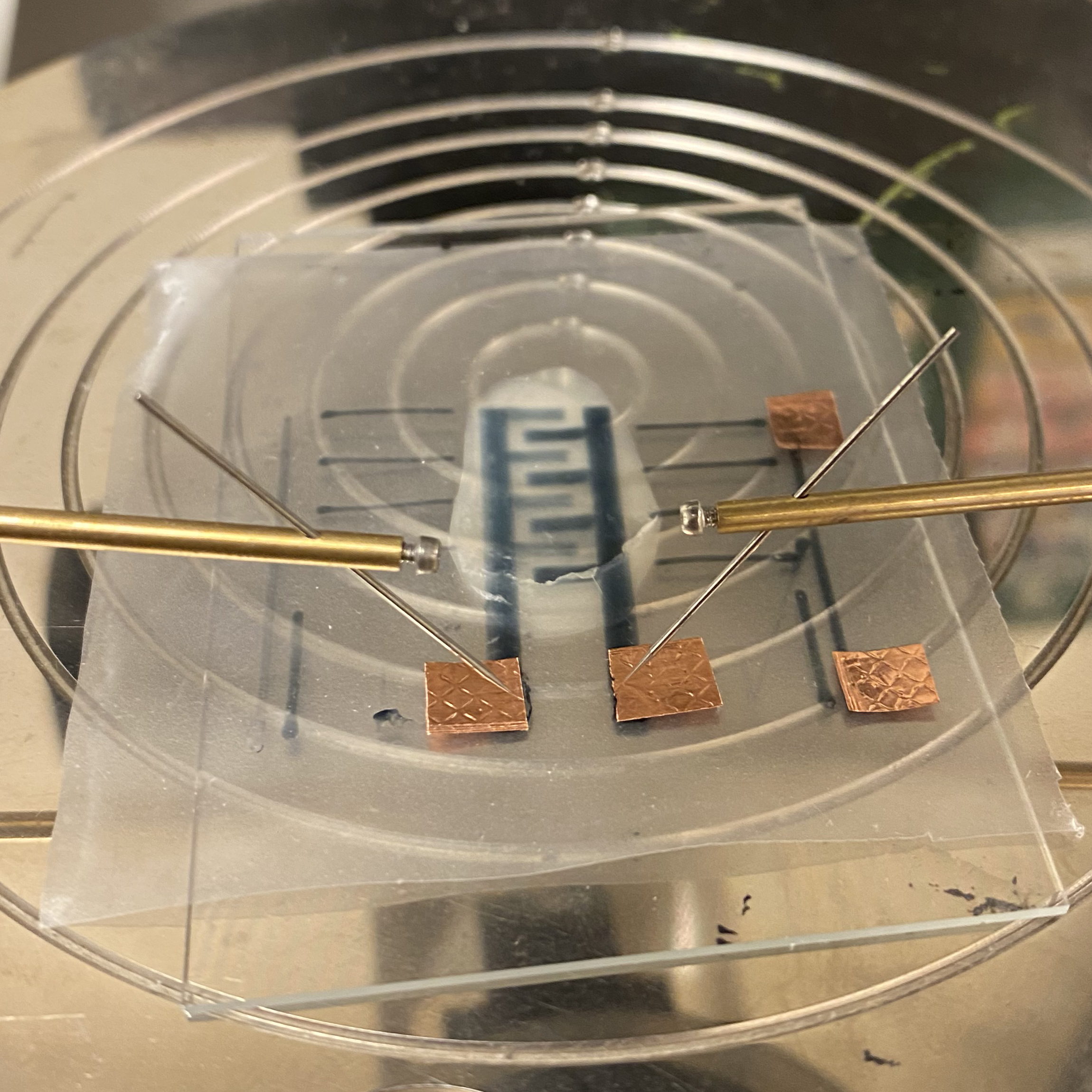
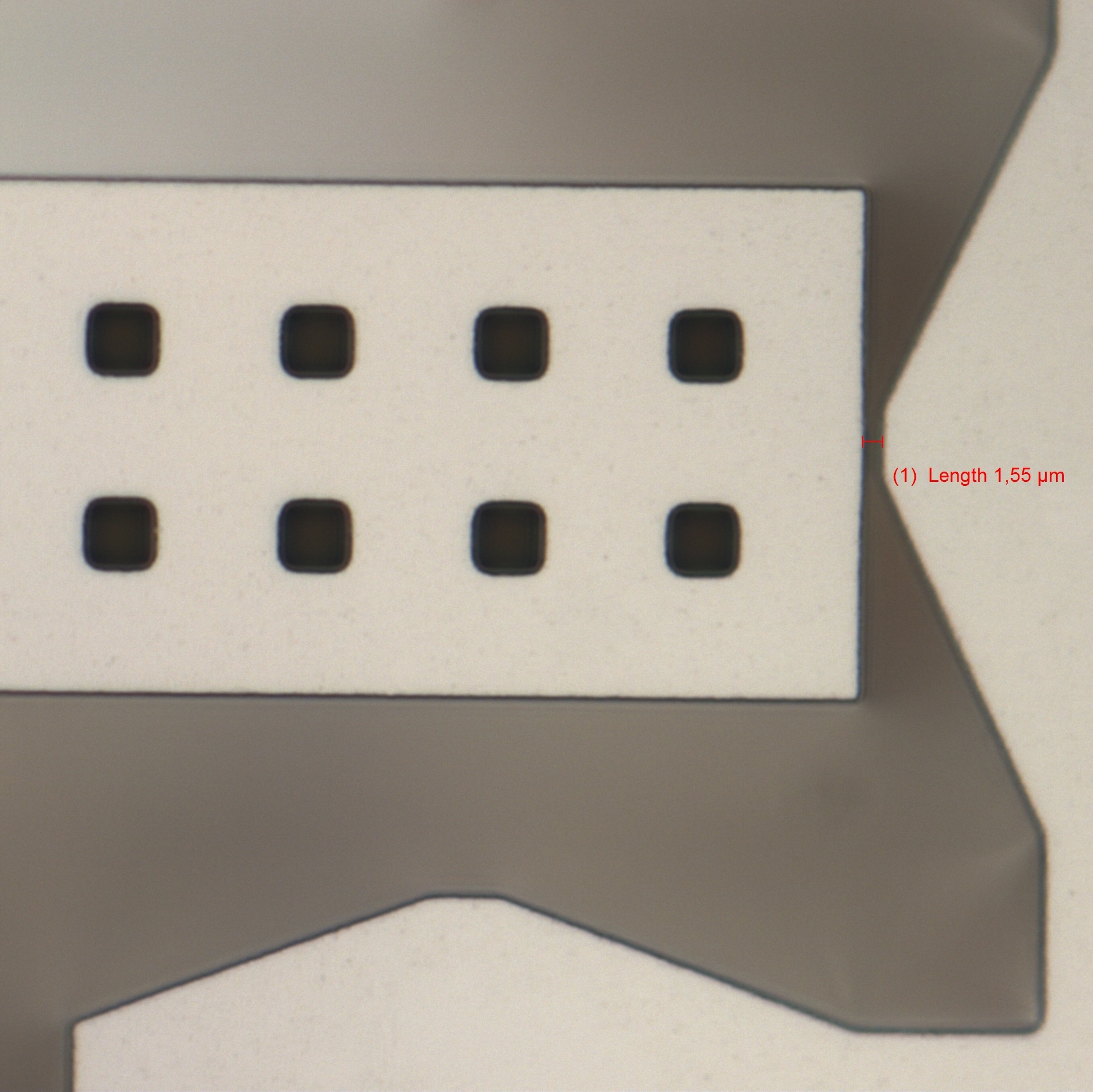
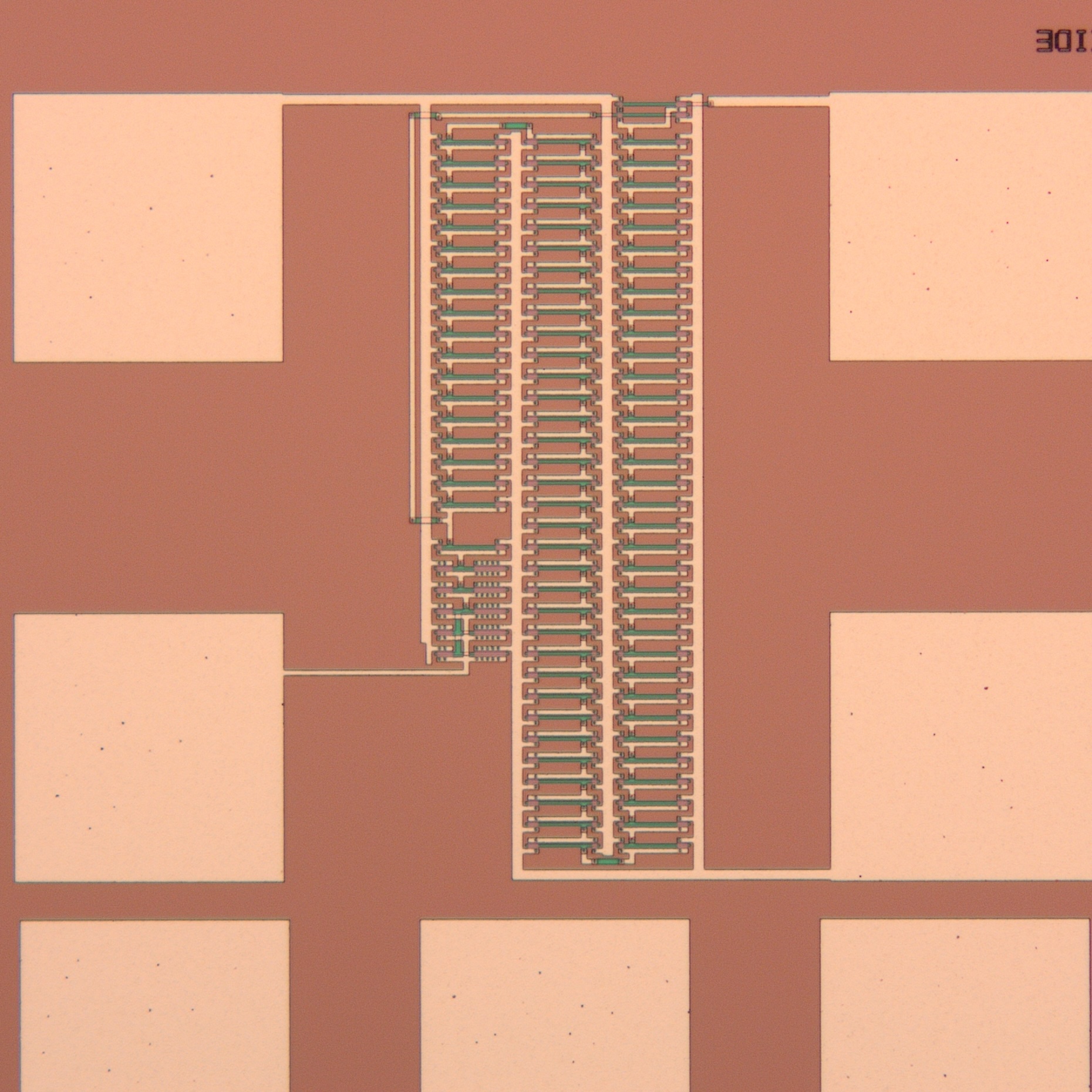
Hollow microneedles
Microneedles made from monocrystalline silicon are at the core of Ascilion's dISF sampling solution. Multiple hollow microneedles, fabricated to form a
precisely engineered pattern, are combined with a capillary system that enables robust sampling and collection of the fluid. With a needle length of less
than 0.5 mm, the sampling process is truly minimally invasive. All needles are connected using an integrated capillary system that drives the fluid towards
a collection reservoir. The capillaries are manufactured for optimal fluidic properties by a combination of deep reactive ion etching of silicon and anodic
bonding of glass utilizing state of the art MEMS processing.
The PELSA system
The PELSA system consists of a sampling tool, a monitoring system, and a PC-controlled vacuum unit. Sampling is controlled
from the PC through pre-defined sequences and on-screen visual monitoring of the process. The sampling tool consists of a sterile
and single-use needle unit that docks into a holder. Together with a visual inspection system, an easy-to-use tool is formed intended
for vacuum aided sampling of dermal interstitial fluid. The tool can either be demounted for direct access to the sampled fluid using
a pipette or a storage capillary for collection of the fluid after sampling. The system, with Ascilion's fifth generation hollow microneedles
chip at the core and a wealth of pre-clinical test data, is well positioned to enter clinical studies both in Europe and the US.
Contributions to the project
- Led the development of PELSA from prototype to a market-ready medical device
- Led the method development for optimal usage of PELSA
- Design, fabrication and inspection of MEMS components
- Designed and implemented test setups for various components, including mechanical and optical
- Developed software using SQL, C#, C++, and Python for lab equipment, data acquisition, and analysis
- Created the main GUI for PELSA, enabling user-friendly device control and data visualization
- Designed and built a fully automated optical inspection tool for quality assurance
- Programmed a 4-axis stage system and implemented real-time image analysis
- Conducted and designed experiments in microfluidics and biology
- Managed incoming inspection of silicon wafers using SEM and LOM
- Designed and manufactured customized tools for wafer inspection
- Contributed to project management, product design, and documentation
- Assisted with regulatory compliance work for medical devices
- Provided customer support and training for the PELSA system
What I learned from this project:
This role provided me with invaluable experience in medical device development, from initial concept to market-ready
product.
On the hardware side, I significantly expanded my skills in designing and building complex systems. I gained
hands-on experience
with various sensors, actuators, and microcontrollers, learning to integrate them into cohesive, functional units.
My proficiency in
PCB design and prototyping grew substantially, as did my understanding of MEMS design and processing.
I became adept at designing and implementing robust test setups for validating hardware components,
enhancing my problem-solving
skills in real-world engineering challenges.
My expertise in CAD (Solidworks) deepened, allowing me to design intricate parts for both prototyping and
production. I gained
practical knowledge in 3D printing and plastic injection molding, understanding the nuances of designing for
manufacturability. In the
realm of microfluidics, I learned to design and fabricate complex microfluidic chips, gaining insights into fluid
dynamics at the microscale.
On the software front, I significantly expanded my programming skills, teaching myself advanced development
techniques across
multiple languages. I learned to create intuitive GUIs and robust backend systems, integrating hardware controls
with user-friendly interfaces.
My experience in data acquisition, analysis, and visualization grew substantially, enabling me to extract meaningful
insights from complex datasets.
Working in a startup environment taught me the importance of versatility, quick problem-solving, and taking
on multiple
responsibilities. I learned to navigate the complexities of regulatory compliance in the medical device industry,
gaining valuable
insights into quality assurance and documentation processes. This experience also honed my project management skills
and taught me how
to effectively communicate technical concepts to diverse audiences, from fellow engineers to customers.
Overall, this role not only enhanced my technical abilities across both hardware and software domains but
also developed my
capacity to thrive in a fast-paced, innovative environment where adaptability and continuous learning are key. It
provided me with a comprehensive
understanding of the entire product development lifecycle in the medical device industry.

The PELSA dISF extraction system
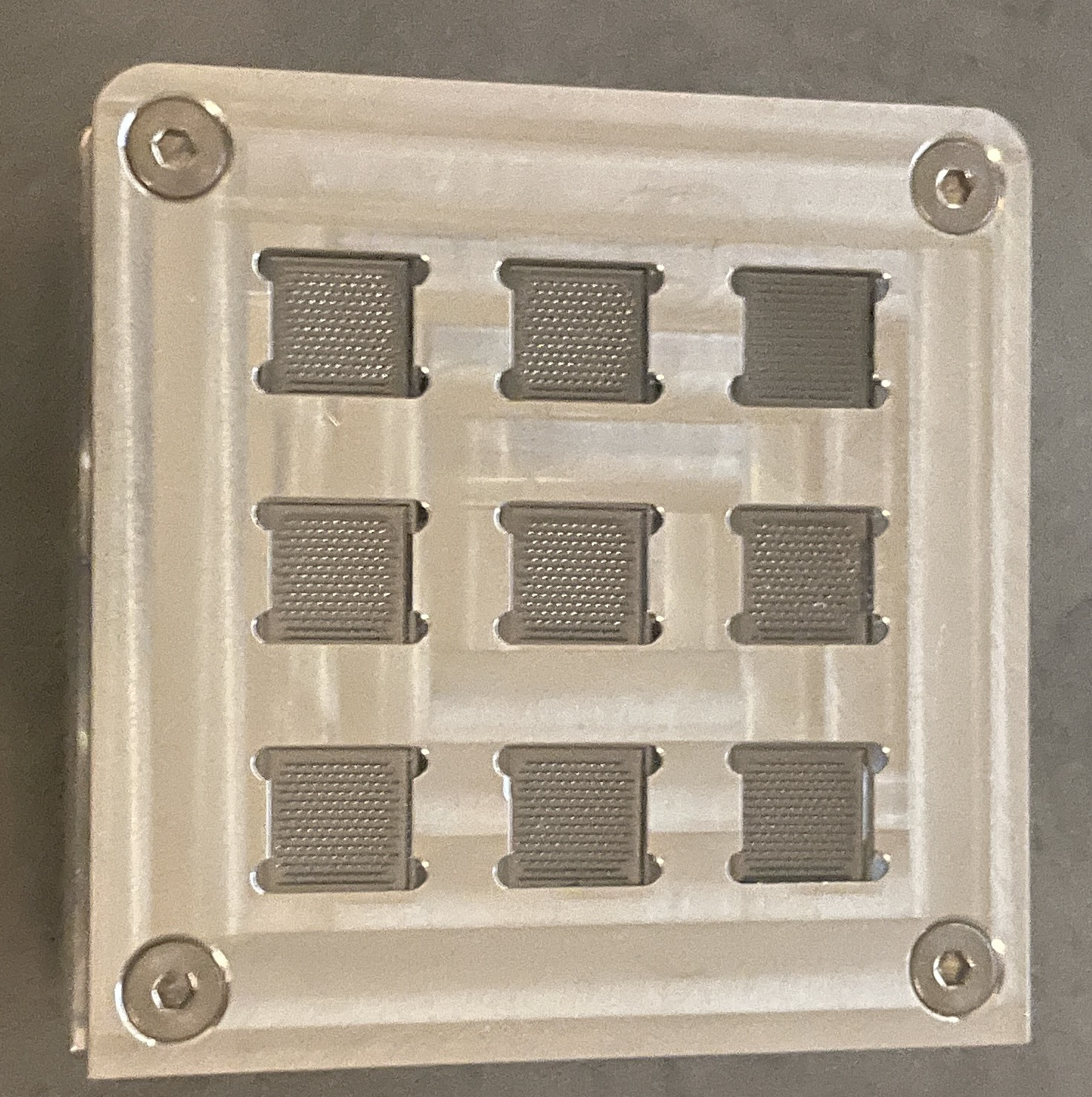
Custom-made chip holder for SEM-inspection